User:Gasmasque/sandbox2
| Gasmasque/sandbox2 | |
|---|---|

| |
| FHPR L2003-2, a Helicoprion davisii tooth-whorl from the Phosphoria Formation of Idaho, Utah Field House of Natural History | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Chondrichthyes |
| Subclass: | Holocephali |
| Order: | †Eugeneodontida |
| Family: | †Helicoprionidae |
| Genus: | †Helicoprion Karpinsky, 1899 |
| Type species | |
| Helicoprion bessonowi Karpinsky, 1899
| |
| Other species | |
| |
| Synonyms | |
|
Synonyms of H. davisii
Synonyms of H. bessonowi
Indeterminate species
| |
Helicoprion is an extinct genus of shark-like[1] cartilaginous fish in the order Eugeneodontiformes. Almost all Helicoprion fossils consist of spirally-arranged clusters of fused teeth, called "tooth whorls", which in life were embedded in the lower jaw. With the exception of the upper and lower jaws, the cartilaginous skeleton of Helicoprion is unknown. The closest living relatives of Helicoprion (and other eugeneodonts) are the chimaeras, though their relationship is very distant. The unusual tooth arrangement is thought to have been an adaption for feeding on soft-bodied prey, and may have functioned as a deshelling mechanism for hard-bodied cephalopods such as nautiloids and ammonoids. In 2013, study of the genus Helicoprion via morphometric analysis of the tooth whorls found that the genus contained only the species H. davisii, H. bessonowi and H. ergassaminon.
Fossils of Helicoprion have been found worldwide, with the genus being known from Russia, Australia, China, Kazakhstan, Japan, Laos, Norway, Canada, Mexico, and the United States (Idaho, Nevada, Wyoming, Texas, Utah, and California).
Discovery and research history
[edit]Valid species
[edit]
While many species of Helicoprion have been described and many fossil specimens have been assigned to the genus, only three species are currently recognized as valid based on morphometric analyses; Helicoprion davidsii, H. bessonowi and H. ergassaminon. These species are known from a 20 million-year timespan during the Permian period, from the Artinskian stage of the Cisuralian (Early Permian) to the Roadian stage of the Guadalupian (Middle Permian). More than 50% of the fossils referred to Helicoprion are H. davisii specimens from the Phosphoria Formation of Idaho. An additional 25% of fossils are H. bessonowi specimens found in the Ural Mountains of Russia.[2]
The type species,[3] Helicoprion bessonowi, was first described in an 1899 monograph by Alexander Karpinsky.[4][5] The first remains of the species to be identified were sent to Karpinsky the year prior by inspector A. Bessinov, after whom the species is named.[5][4][6]: 76–80 Although it was not the first Helicoprion species to be discovered, it was the first to be described from complete tooth whorls, and its discovery established that Helicoprion was a distinct genus.[5] H. bessonowi is primarily based on a number of specimens from Artinskian-age limestone of the Divya Formation, in the Ural Mountains of Russia. H. bessonowi specimens are also known from the Tanukihara Formation of Japan and Artinskian-age strata in Kazakhstan.[7][8] In 1999, the holotype of H. bessonowi was stolen, but afterwards was shortly recovered with the aid of an anonymous fossil dealer.[9][10]
The first specimen of Helicoprion to be described was WAMAG 9080,[2] a 15-tooth fragment of a tooth whorl found along a tributary of the Gascoyne River in Western Australia. Henry Woodward described the fossil in 1886 and named it as a species of Edestus: E. davisii, commemorating its discoverer, a gold prospector whose last name was Davis.[6]: 6–7 [11][note 1] Upon naming H. bessonowi in 1899, Alexander Karpinsky reassigned E. davisii as another species of Helicoprion.[5][10] In 1902, Charles R. Eastman instead referred H. davisii to his genus Campyloprion, but this proposal was never widely accepted.[12] Karpinsky's identification of Edestus davisii as a species of Helicoprion would eventually be upheld by Curt Teichert, who discovered several more complete tooth whorls from the Wandagee Formation of Western Australia in 1939 and described them in 1940.[6]: 127–129 [13] Outside of Western Australia and the Phosphoria Formation of Idaho, H. davisii specimens have also been found in Mexico, Texas, and Canada (Nunavut and Alberta).[2]

Helicoprion ergassaminon is known from the Phosphoria Formation, and is very rare compared to the cohabitating H. davidsii. H. ergassaminon was named and described in detail within a 1966 monograph by Svend Erik Bendix-Almgreen, and the holotype specimen ("Idaho 5"), bears breakage and wear marks indicative of its usage in feeding. H. ergassaminon is also represented by several other specimens from the Phosphoria Formation, though none of these show wear marks.[14] The type specimen of this species was formerly considered lost, but was identified in 2017 and, in 2023, was returned to the collection of the Idaho Museum of Natural History.[10][15]
Synonymous species
[edit]
In 1907 and 1909, Oliver Perry Hay described a new genus and species of eugeneodont, Lissoprion ferrieri, from numerous fossils found in phosphate-rich Phosphoria Formation on the border between Idaho and Wyoming. He also synonymized H. davisii with his new genus and species.[16] However, Karpinsky separated the two species once more and transferred them to Helicoprion in 1911.[17] H. ferrieri was initially differentiated using the metrics of tooth angle and height, but Tapanila and Pruitt (2013) considered these characteristics to be variable within a single Helicoprion species. As a result, they reassigned H. ferrieri as a junior synonym of H. davisii.
In a 1939 publication, Harry E. Wheeler described two new species of Helicoprion from California and Nevada. One of these, H, sierrensis, was described from a specimen (UNMMPC 1002) found in glacial moraine deposits in Eastern California, likely originating from the Goodhue Formation. The other species, H. nevadensis, is based on a single partial fossil found in a Nevadan mine by Elbert A. Stuart in 1929.[18] This fossil, UNMMPC 1001, has been lost. It was reported as having originated from the Rochester Trachyte deposits, which Wheeler considered to be of Artinskian age. However, the Rochester Trachyte is considered to be of Triassic age, and H. nevadensis likely did not originate in the Rochester Trachyte, rendering its true age unknown. Wheeler differentiated H. nevadensis from H. bessonowi by its pattern of whorl expansion and tooth height, but Tapanila and Pruitt showed in 2013 that these were consistent with H. bessonowi at the developmental stage that the specimen represents. Tapanila and Pruitt also determined that the distinguishing shaft range of H. sierrensis was well within the variation found in the species H. davisii.[2]
Based on isolated teeth and partial whorls found on the island of Spitsbergen, Norway, researcher Stanisław Siedlecki described H. svalis in 1970. The type specimen, a very large whorl with specimen number PMO A-33961, was noted for its narrow teeth that apparently are not in contact with each other,[19] but this seems to be a consequence of only the central part of the teeth being preserved, according to Tapanila and Pruitt. Since the whorl shaft is partially obscured, H. svalis cannot be definitely assigned to H. bessonowi, but it closely approaches the latter species in many aspects of its proportions. With a maximum volution height of 72 mm (2.8 in), H. svalis is similar in size to the largest H. bessonowi, which has a maximum revolution height of 76 mm (3.0 in).[2]
H. jingmenense was described in 2007 from a nearly complete tooth whorl (YIGM V 25147) with more than four revolutions across a part and counterpart slab. It was discovered during the construction of a road passing through the Lower Permian Qixia Formation of Hubei Province, China. The specimen is very similar to H. ferrieri and H. bessonowi, though it differs from the former by having teeth with a wider cutting blade, and a shorter compound root, and differs from the latter by having fewer than 39 teeth per volution.[20] Tapanila and Pruitt argued that the specimen was partially obscured by the surrounding matrix, resulting in an underestimation of tooth height. Taking into account intraspecific variation, they synonymized it with H. davisii.[2]
Other species
[edit]
Several large whorls are difficult to assign to any particular species group, similarly to H. svalis. IMNH 14095, a specimen from Idaho, appears to be similar to H. bessonowi, but it has unique flange-like edges on the apices of its teeth. IMNH 49382, also from Idaho, has the largest known whorl diameter at 56 cm (22 in) for the outermost volution (the only one preserved), but it is incompletely preserved and still partially buried.[2] H. mexicanus, named by F.K.G. Müllerreid in 1945, was supposedly distinguished by its tooth ornamentation. Its holotype is currently missing, though its morphology was similar to that of IMNH 49382. In the absence of other material, it is currently a nomen dubium. Various other indeterminate Helicoprion specimens have been described from Canada, Japan, Laos,[21] Idaho, Utah, Wyoming, and Nevada.[3][2]
In 1916, Karpinsky named the species H. clerci for several fragments of a very large tooth whorl found in the Divya Formation.[6]: 121 [22][23] In 1924, Karpinsky separated H. clerci from Helicoprion and reclassified it under the new genus, Parahelicoprion.[24][25] P. clerci has been suggested to represent a junior synonym of Helicoprion by paleontologist Serge Naugolnykh,[22] although other authors retain this species in its own genus.[26] In 1922, Karpinsky named the new species Helicoprion ivanovi, from Gzhelian (latest Carboniferous) strata near Moscow.[27] However, this species was subsequently removed from Helicoprion and placed as a second species of the related eugeneodont Campyloprion,[28] and more recently it has become the type species of the genus Karpinskiprion.[10][26] Vladimir Obruchev described H. karpinskii from two teeth in 1953.[29] He provided no distinguishing traits for this species, thus it must be regarded as a nomen nudum.[2] Various other indeterminate Helicoprion specimens have been described from Canada, Japan, Laos, Idaho, Utah, Wyoming, and Nevada.[2][3][21]
Description
[edit]Like other chondrichthyan fish, Helicoprion had a skeleton made of cartilage. Around the jaws this cartilage was mineralized,[14][8] but the rest of the skeleton was likely unmineralized and quickly disintegrated once it began to decay.[30] This makes drawing precise conclusions on the in-life appearance of Helicoprion difficult, but the body shape has been estimated from postcranial remains known from other members of its order,[8][31][32] such as the Pennsylvanian to Triassic-age caseodontids Caseodus, Fadenia, and Romerodus.[30][33]
The caseodontids have a fusiform (streamlined, torpedo-shaped) body plan, with triangular pectoral fins. They have a single large and triangular dorsal fin without a fin spine, and a tall, forked caudal fin, which externally appears to be homocercal (with two equally sized lobes).[30] This general body plan is shared by active, open-water predatory fish such as tuna, swordfish, and lamnid sharks.[8][34] Eugeneodonts also lack pelvic and anal fins, and the genus Romerodus had broad keels along the side of the body up to the caudal fin.[30] Fadenia and the smaller Ornithoprion had at least five well-developed gill slits, possibly with a vestigial sixth gill.[31][33][35] No evidence has been found of the specialized gill basket and fleshy operculum present in living chimaeroids.[30][33] Based on the proportional size of caseodontoid tooth whorls, researcher Oleg Lebedev suggested that Helicoprion individuals with tooth whorls 35–40 cm (14–16 in) in diameter could reach 5–8 m (16–26 ft) in total length, rivaling the size of modern basking sharks.[8] The largest known Helicoprion tooth-whorl, specimen IMNH 49382 representing an unknown species, reached 56 cm (22 in) in diameter and 14 cm (5.5 in) in crown height, and would have belonged to an individual 7 m (23 ft) or more in length.[2][32][36]
Tooth whorls
[edit]
Almost all Helicoprion specimens are known solely from "tooth whorls", which consist of dozens of enameloid-covered teeth embedded within a common logarithmic spiral-shaped root. The youngest and first tooth at the center of the spiral, referred to as the "juvenile tooth arch", is hooked, but all other teeth are generally triangular in shape, laterally compressed and typically serrated.[36] Tooth size increases away from the center of the spiral (abaxial), with the largest teeth possibly exceeding 10 cm (3.9 in) in length. The lower part of the teeth form projections that are shingled below the crown of the previous tooth. The lowest portion of the root below the enameloid tooth projections is referred to as the "shaft", and lies on cartilage that encapsulates the previous revolutions of the whorl. In a complete tooth-whorl, the outermost part of the spiral terminates with an extended section of shaft that lacks the middle and upper portions of the tooth crown.[2]
The three species of Helicoprion differ only in the anatomy of their tooth-whorls. Each species is differentated by features of the upper, middle and lower sections of the tooth crowns, which are apparent only after the 85th tooth of the spiral. H. davisii can be differentiated from other Helicoprion species by a short and narrowly spaced tooth whorl, backward-curved tooth tips, obtusely angled tooth bases, and a consistently narrow whorl shaft.[2] Helicoprion ergassaminon is roughly intermediate in anatomy between H. bessonowi and H. davisii, having tall but narrowly spaced teeth. Its teeth are also gently curved, with obtusely angled tooth bases.[31]
Cartilaginous skull
[edit]
Helicoprion specimens preserving more than tooth whorls are rare. The best-preserved specimen of Helicoprion is IMNH 37899 (also known as "Idaho 4"), referred to Helicoprion davisii. It was found in Idaho in 1950 and was originally described in 1966 by paleontologist Svend Erik Bendix-Almgreen.[14] A 2013 redescription by Tapanila and colleagues was accompanied by CT scanning, which allowed for more detailed study of the specimen's cartilage. CT scanning revealed a nearly complete set of upper and lower jaws, still in articulation and preserved in three dimensions. Alongside the tooth-whorl, the specimen preserves the palatoquadrates (forming the upper jaw), Meckel's cartilages (forming the lower jaw), and a robust block of cartilage bracing the tooth-whorl which has been identified as labial cartilage. All of these structures are mineralized and covered in prismatic calcified cartilage, as in modern cartilaginous fish. The specimen do not preserve a chondrocranium, the cartilaginous structure which would have housed the brain and sensory organs. The jaws are extensively laterally compressed (narrow) compared to living chondrichthyans, though this may at least partially be an artifact of post mortem (after death) compression.[31][37]

Helicoprion had an autodiastylic jaw suspension, meaning that the inner edge of the palatoquadrate was firmly attached (but not fused) to the chondrocranium at two separate points. These two attachment points are the dome-shaped ethmoid process at the front of the palatoquadrate, and the flange-like basal process at its upper rear corner.[31] Autodiastylic jaws are common in early holocephalans, though in modern animals they can only be found in embryonic chimaeriforms.[38] Another well-preserved specimen, USNM 22577+494391 (nicknamed the "Sweetwood specimen"), has demonstrated that the inner surface of the palatoquadrate was covered with numerous small (~2 mm wide) teeth.[36] The palatoquadrate teeth were low and rounded, forming a "pavement" that scraped against the tooth whorl.[37] When seen from behind, the palatoquadrate forms a paired jaw joint with the Meckel's cartilage. No evidence is seen for an articulation between the palatoquadrate and the hyomandibula.[31]
Meckel's cartilage has an additional projection right before the joint with the palatoquadrate. This extra process, unique to Helicoprion, likely served to limit jaw closure to prevent the whorl from puncturing the chondrocranium. Another unique characteristic of Helicoprion is that the preserved labial cartilage forms a synchondrosis (fused joint) with the upper surface of Meckel's cartilage. This joint is facilitated by a long facet on the upper edge of Meckel's cartilage. The labial cartilage provides lateral support for the tooth whorl, widening near the root of each revolution. By wedging into the palatoquadrate while the mouth is closed, the upper edge of the labial cartilage helps to spread out the forces used to limit the extent of the jaw closure. The rear portion of the labial cartilage has a cup-like form, protecting the developing root of the last and youngest revolution.[31][37][36]
Scales
[edit]Tooth-like chondricthyan scales, specifically known as odontodes, have been found associated with H. bessonowi remains in Kazakhstan. They are broadly similar to scales of other eugeneodonts such as Sarcoprion and Ornithoprion.[8][35] The scales have a cap-shaped base with a concave lower surface. The crowns are conical and covered with serrated, longitudinal ridges. The scales may be monodontode (with one crown per base) or polyodontode (with multiple crowns resulting from the fusion of several odontodes). Compared to other eugeneodonts, the scales of Helicoprion are more strongly pointed.[8]
Classification
[edit]Skull data from IMNH 37899 reveal several characteristics, such as an autodiastylic (two-jointed) jaw suspension with a non-suspensory hyomandibula, which confirm the placement of Helicoprion within the chondrichthyan subclass Holocephali (or the more broadly defined Euchondrocephali). Holocephalans are primarily an extinct group, and the only extant representatives of the group are the specialized, deep-sea Chimaeriformes (also called ratfish).[39] The relationship between Helicoprion and living chimaeras is very distant, but had been previously suspected based on details of its tooth anatomy.[31]
Helicoprion can be characterized as a member of Eugeneodontiformes, an order of holocephalans that lived from the Devonian to Triassic periods and are defined by their tooth-whorls along the midline of the jaw and autodiastylic skulls.[30][31][36] Within the Eugeneodontiformes, Helicoprion is placed within the Edestoidea, a group of eugeneodonts with particularly tall and angled symphysial teeth. Members of the Edestoidea are divided into two families based on the style of the dentition. One family, the Edestidae, has relatively short tooth blades with roots that incline backwards.[30][36] The other family, which contains Helicoprion, is sometimes called Agassizodontidae, based on the genus Agassizodus.[30][36] Other authors, though, prefer the family name Helicoprionidae, which was first used 70 years prior to Agassizodontidae. Helicoprionids (or agassizodontids) have large, cartilage-supported whorls with strongly arched shapes. Helicoprionids do not shed their teeth; instead, their tooth whorls continually add new teeth with bases inclined forwards at the top of the whorl.[30][8] As most eugeneodonts are based on fragmentary tooth remains, concrete phylogenetic relationships within the group remain unclear.[36] A cladogram illustrating the group's possible relations, drawn from Rainer Zangerl's 1981 volume of the Handbook of Paleoichthyology,[30] is provided below.
| Eugeneodontida (=Eugeneodontiformes) |
| |||||||||||||||||||||||||||
Paleobiology and whorl function
[edit]
The unusual saw-like tooth whorl and the lack of wear on the teeth of Helicoprion implies a diet of soft-bodied prey, as hard-shelled prey would simply slip out of the mouth. Due to the narrow nature of the jaw, suction feeding is unlikely to have been effective, and Helicoprion is thought to have been a bite feeder. Biomechanical modelling by Ramsay et al. (2015) suggests that the teeth in the whorl had distinct functions depending on where they were in the spiral. The frontmost teeth served to snag and pull prey further into the mouth, while the middle teeth spear, and the hind teeth served to puncture and bring prey further into the throat, with the prey being squeezed between the whorl and the two halves of the palatoquadrate. The labial cartilage served to buttress and provide support to the whorl.[37]

Helicoprion may have started with a large gape during initial prey capture, followed by smaller jaw opening and closing cycles to further transport prey into the mouth, as is done by modern bite-feeding sharks. While modern sharks shake their heads from side to side to facilitate sawing and cutting their prey, the teeth of Helicoprion would likely further cut the prey during the jaw opening, due to the arc-like path of the front teeth, similar to the slashing motion of a knife. Helicoprion likely used a series of rapid, forceful jaw closures to initially capture and push prey deeper into the oral cavity, followed by cyclic opening and closing of the jaw to facilitate sawing through prey.[37]
Ramsay and colleagues further suggested that the whorl could have served as an effective mechanism for deshelling hard-shelled cephalopods such as ammonoids and nautiloids, which were abundant in Early Permian oceans. If a hard-shelled cephalopod was bitten head-on, the whorl could have served to pull the soft body out of the shell and into the mouth. During jaw closure, the palatoquadrates and tooth whorl combined to form a three-point system, equivalent to the set-up of an inverted three-point flexural test. This system was effective at trapping and holding soft parts to increase cutting efficiency and provide leverage against hard-shelled prey. At the three points of contact, the estimated bite force ranges between 1,192 and 2,391 newtons (268 and 538 lbf), with estimated bite stresses ranging from 397 to 797 million N/m2 (57,600 to 115,600 psi) during initial prey contact. This large bite force may have allowed Helicoprion to expand its diet to vertebrates, as its jaw apparatus was more than capable of cutting through skeletal elements of unarmoured bony fish and other chondrichthyans.[37]
Historical reconstructions
[edit]Earliest reconstructions
[edit]
Hypotheses for the placement and identity of Helicoprion's tooth whorls were controversial since it was first discovered. Henry Woodward, who referred the first known Helicoprion fossils to Edestus, discussed the various hypotheses concerning the nature of Edestus fossils. Joseph Leidy, who originally described Edestus vorax, argued that they represented the jaws of "plagiostomous" (chondrichthyan) fish. William Davies agreed, specifically comparing it to the jaws of Janassa bituminosa, a Permian petalodont. Researcher J.S. Newberry alternatively suggested that the jaw-like fossils were defensive spines of a stingray-like fish. Woodward eventually settled on E.D. Cope's argument that they represented pectoral fin spines from fish similar to Pelecopterus (now known as Protosphyraena; then sometimes assigned to Ptychodus).[11]
Karpinsky's 1899 monograph on Helicoprion noted that the bizarre nature of the tooth whorl made reaching precise conclusions on its function difficult. He tentatively suggested that it curled up from the upper jaw for defensive or offensive purposes. This was justified by comparison to the upper tooth blades of Edestus, which by 1899 had been re-evaluated as structures belonging to the jaw.[5]
Debates over the identity of Helicoprion's tooth whorl were abundant in the years following Karpinsky's monograph. In 1900, the publication was reviewed by Charles Eastman, who appreciated the paper as a whole, but derided the sketch of the supposed life position of the whorl. Though Eastman admitted that the teeth of the whorl were very similar to those of other chondrichthyans, he still supported the idea that the whorl may have been a defensive structure embedded into the body of the animal, rather than the mouth.[40] Shortly after his original monograph, Karpinsky published the argument that the whorl represented a curled, scute-covered tail akin to that of Hippocampus (seahorses).[41] This proposal was immediately criticized by various researchers. E. Van den Broeck noted the fragility of the structure and argued that it was most well-protected as a paired feeding apparatus in the cheek of the animal.[42] A.S. Woodward (unrelated to Henry Woodward) followed this suggestion with the hypothesis that each whorl represented a tooth battery from a gigantic shark.[43] G. Simoens illustrated Karpinsky's various proposals and used histological data to adamantly argue that the whorls were toothed structures placed within the mouth.[44] In 1911, Karpinsky illustrated the whorls as components of the dorsal fins.[17] Reconstructions similar to those of Karpinsky (1899) were common in Russian publications as late as 2001.[8]
Later reconstructions
[edit]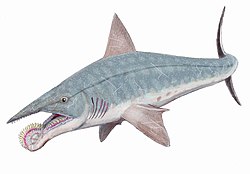
By the mid-20th century, the tooth whorl was generally accepted as positioned in the lower jaw of the animal. Though this general position was suspected almost immediately in the aftermath of Karpinsky's monograph, it was not illustrated as such until the mid-1900s. Around that time, an artist known only as "F. John" depicted Helicoprion within a set of "Tiere der Urwelt" trading cards. Their reconstruction presented the tooth whorl as an external structure curling down from the lower jaw of the animal. Similar downward-curling reconstructions have also been created by modern paleontologists and artists such as John A. Long, Todd Marshall, and Karen Carr. The utility of the tooth whorl in this type of reconstruction was inferred based on sawfish, which incapacitate prey using lateral blows of their denticle-covered snouts.[8][31]
The other publication was Bendix-Almgreen's monograph on Helicoprion. His investigations reinterpreted the tooth whorl as a symphyseal structure wedged between the meckelian cartilages, which were separated by a gap at the front. A pair of cartilage loops, the symphyseal crista, seems to develop as a paired extension of the jaw symphysis where the meckelian cartilages meet at the back of the jaw. Each loop arches up before curling back inwards, tracing over the root of the tooth whorl. The largest and youngest teeth form at the symphysis near the back of the jaw. Over time, they are carried along the symphyseal crista, spiraling forwards, then downwards and inwards. The series of teeth accumulates into a spiraling structure, which is housed within the cavity defined by the symphyseal crista. The lateral and lower edges of the tooth whorl would have been obscured by skin during life. According to Bendix-Almgreen, the most likely use of the tooth whorl was as a tool for tearing and cutting prey against the upper jaw.[14]
In the 1994 book Planet Ocean: A Story of Life, the Sea, and Dancing to the Fossil Record, author Brad Matsen and artist Ray Troll describe and depict a reconstruction based on the information gleaned by Bendix-Almgreen (1966). They proposed that no teeth were present in the animal's upper jaw, besides crushing teeth for the whorl to cut against. The two envisioned the living animal to have a long and very narrow skull, creating a long nose akin to the modern-day goblin shark.[45] A 1996 textbook by Philippe Janvier presented a similar reconstruction, albeit with sharp teeth at the front of the upper jaw and rows of low crushing teeth in the back of the jaw.[46]
In 2008, Mary Parrish created a new reconstruction for the renovated Ocean Hall at the Smithsonian Museum of Natural History. Designed under the direction of Robert Purdy, Victor Springer, and Matt Carrano, Parrish's reconstruction places the whorl deeper within the throat. This hypothesis was justified by the argument that the teeth supposedly had no wear marks, and the assumption that the whorl would have created a drag-inducing bulge on the chin of the animal if located in a symphysial position. They envisioned the tooth whorl as a structure derived from throat denticles and designed to assist swallowing. This would hypothetically negate the disadvantages the tooth whorl would produce if positioned further forward in the jaw.[47] This reconstruction was criticized for the overly intricate and potentially ineffective design of such a structure, if solely used to assist swallowing.[48]
Lebedev (2009) found more support for a reconstruction similar to those of Bendix-Almgreen (1966) and Troll (1994).[8] A tooth whorl found in Kazakhstan preserved radial scratch marks; the whorl was also found near several wide, tuberculated teeth similar to those of the putative caseodontoid Campodus. Lebedev's reconstruction presented a cartilage-protected tooth whorl in a symphysial position at the front of the long lower jaw. When the mouth was closed, the tooth whorl would fit into a deep longitudinal pocket on the upper jaw. Both the pocket in the upper jaw and the edges of the lower jaw would have been lined with dense rows of Campodus-like teeth. This was similar to the situation reported in related helicoprionids such as Sarcoprion and Agassizodus. As for Helicoprion's ecology, it was compared to modern cetaceans such as Physeter (the sperm whale), Kogia (dwarf and pygmy sperm whales), Grampus (Risso's dolphin), and Ziphius (Cuvier's beaked whale). These fish- and squid-eating mammals have reduced dentition, often restricted to the tip of the lower jaw.[8] Lebedev's reconstruction approximates modern views on Helicoprion's anatomy, though the hypothetical long jaw and Campodus-like lateral dentition has been superseded by CT data.[31]
Paleoecology
[edit]During the Artinskian the Phosphoria Formation represented a deep inland sea with an anoxic, muddy bottom. Based on the large number of specimens discovered in the formation, the Phosphoria Sea may have been either a nursery for breeding Helicoprion, or a productive hunting ground.[6]: 116–117
Helicoprion is believed to have been the apex predator of its environment.[22]
Extinction
[edit]The genus became extinct during the Guadalupian stage of the Middle Permian. It is not associated with any larger extinction event, and has been suggested to be a "background extinction"
Notes
[edit]- ^ The full name of Mr. Davis is unknown
References
[edit]- ^ Viegas, Jennifer (February 27, 2013). "Ancient shark relative had buzzsaw mouth". science.nbcnews.com.
- ^ a b c d e f g h i j k l m Tapanila, L.; Pruitt, J. (2013). "Unravelling species concepts for the Helicoprion tooth whorl" (PDF). Journal of Paleontology. 87 (6): 965–983. Bibcode:2013JPal...87..965T. doi:10.1666/12-156. S2CID 53587115. Archived from the original (PDF) on 2016-06-01. Retrieved 2017-07-26.
- ^ a b c Larson, E. R.; Scott, J. B. (1955). "Helicoprion from Elko County, Nevada". Journal of Paleontology. 29 (5): 918–919. JSTOR 1300414.
- ^ a b W, A. S. (1900). "II.—Helicoprion—Spine or Tooth? - "Ueber die Reste von Edestiden und die neue Gattung Helicoprion." By A. Karpinsky. Verhandl. k. russ. min. Ges. St. Petersburg, ser. ii, vol. xxxvi, No. 2, with 4 pls. and 72 text-figs. (1899)". Geological Magazine. 7 (1): 33–36. doi:10.1017/S0016756800159886. ISSN 1469-5081.
- ^ a b c d e Карпинскій, А. (1899). Объ остаткахъ eдестидъ и о новомъ ихъ родѣ Helicoprion [On the edestid remains and the new genus Helicoprion]. Записки Императорской Академіи Наукъ (Notes of the Imperial Academy of Sciences). По Физико-математическому отдѣленіи (Physics and Mathematics section) (in Russian). 8 (7): 1–67; Pl. I–IV.
{{cite journal}}: CS1 maint: postscript (link)
Also printed as "Ueber die Reste von Edestiden und die neue Gattung Helicoprion". Записки Императорскаго С.-Петербургскаго Минералогическаго Обществ(Notes of the Imperial St. Petersburg Mineralogical Society). 2 (in German). 36: 361–476. 1899; Pl. I–IV.{{cite journal}}: CS1 maint: postscript (link) [Reprinted (1899). St. Petersburg: C. Birkenfield. pp. 1–111. urn:nbn:de:bvb:12-bsb00073910-2.] - ^ a b c d e Ewing, Susan (2017). Resurrecting the shark: a scientific obsession and the mavericks who solved the mystery of a 270-million-year-old fossil (1st ed.). New York: Pegasus Books. ISBN 978-1-68177-343-8. OCLC 951925606.
- ^ Yabe, H. (1903). "On a Fusulina-Limeston with Helicoprion in Japan". The Journal of the Geological Society of Japan. 10 (113): 1–13. doi:10.5575/geosoc.10.113_1.
- ^ a b c d e f g h i j k l Lebedev, O.A. (2009). "A new specimen of Helicoprion Karpinsky, 1899 from Kazakhstanian Cisurals and a new reconstruction of its tooth whorl position and function". Acta Zoologica. 90: 171–182. doi:10.1111/j.1463-6395.2008.00353.x. ISSN 0001-7272.
- ^ LONG J. 2002. The Dinosaur Dealers. Mission: to uncover international fossil smuggling. Allen & Unwin, Melbourne, p. 53–57
- ^ a b c d Long, John (2024). The Secret History of Sharks: The Rise of the Ocean's Most Fearsome Predators (1st ed.). New York: Random House Publishing Group. pp. 166–197. ISBN 978-0-593-59807-8.
- ^ a b Woodward, Henry (1886). "On a Remarkable Ichthyodorulite from the Carboniferous Series, Gascoyne, Western Australia". Geological Magazine. New Series, Decade III. 3 (1): 1–7. Bibcode:1886GeoM....3....1W. doi:10.1017/S0016756800144450. S2CID 128724775.
- ^ Eastman, C.R. (April 1902). "II.—On Campyloprion, a New Form of Edestus-like Dentition". Geological Magazine. Decade IV. 9 (4): 148–152. Bibcode:1902GeoM....9..148E. doi:10.1017/S0016756800180926. S2CID 128874659.
- ^ Teichert, Curt (1940). "Helicoprion in the Permian of Western Australia". Journal of Paleontology. 14 (2): 140–149. JSTOR 1298567.
- ^ a b c d Bendix-Almgreen, Svend Erik (1966). "New investigations on Helicoprion from the Phosphoria Formation of south-east Idaho, USA" (PDF). Biol. Skrifter Udgivet Af Det Kongelige Danske Videnskabernes Selskab. 14 (5): 1–54.
- ^ "Fossil Found: Idaho No. 5 Returns to Idaho Museum of Natural History after 60+ Years". isu.edu. 7 September 2023. Retrieved 30 October 2024.
- ^ Hay, Oliver Perry (1909). "On the nature of Edestus and related genera, with descriptions of one new genus and three new species". Proceedings of the United States National Museum. 37 (1699): 43–61. doi:10.5479/si.00963801.37-1699.43. ISSN 0096-3801.
- ^ a b Karpinsky, Alexander (1911). ""Замѣчанія о Helicoprion и о другихъ едестидахъ"". Извѣстія Императорской Академіи Наук = Bulletin de l'Académie Impériale des Sciences de St.-Pétersbourg. VI Серия (in Russian). 5 (16): 1105–1122.
- ^ Wheeler, H. E. (1939). "Helicoprion in the Anthracolithic (Late Paleozoic) of Nevada and California, and its Stratigraphic Significance". Journal of Paleontology. 13 (1): 103–114. JSTOR 1298628.
- ^ Siedlecki, Stanislaw (1968). "A Helicoprion from the Permian of Spitsbergen" (PDF). Årbok Norsk Polarinstitute: 36–54.
- ^ Chen, Xiao Hong; Long, Cheng; Yin, Kai Guo (August 2007). "The first record of Helicoprion Karpinsky (Helicoprionidae) from China". Chinese Science Bulletin. 52 (16): 2246–2251. Bibcode:2007ChSBu..52.2246C. doi:10.1007/s11434-007-0321-y. S2CID 96676181.
- ^ a b Hoffet, J.H. (1933). "Étude géologique sur le centre de l'Indochine entre Tourane et le Mekong". Bulletin de la Service géologique du Indochine. 20: 3–154.
- ^ a b c Naugolnykh, S.V. (2018). "Artinskian (Early Permian) Sea Basin and Its Biota (Krasnoufimsk, Cis-Urals)". Stratigraphy and Geological Correlation. 26 (7): 734–754. Bibcode:2018SGC....26..734N. doi:10.1134/S0869593818070080. S2CID 135304766.
- ^ Karpinsky, Alexander Karpinsky (27 April 1916). "On a new species of Helicoprion (Helicoprion clerci, n. sp.)". Bulletin de l'Académie Impériale des Sciences de Saint Pétersbourg (in Russian) (6): 701–708 – via Biodiversity Heritage Library.
- ^ Karpinsky, Alexander (1922). "Замечания о зубных сегмента х Edestidae и об и х ориентировке (Notes on the dental segments of Edestidae and their orientation)". Bulletin de l'Académie des Sciences de Russie (Bulletin of the Russian Academy of Sciences) (in Russian): 379–388 – via Biodiversity Heritage Library.
- ^ Karpinsky, Alexander (1924). "Helicoprion (Parahelicoprion n.g.) clerci". Записки Уральского общества любителей естествознания (Notes of the Ural Society of Natural Science Lovers). 39: 1–10.
- ^ a b Lebedev, Oleg A.; Itano, Wayne M.; Johanson, Zerina; Alekseev, Alexander S.; Smith, Moya M.; Ivanov, Aleksey V.; Novikov, Igor V. (2023). "Tooth whorl structure, growth and function in a helicoprionid chondrichthyan Karpinskiprion (nom. nov.) (Eugeneodontiformes) with a revision of the family composition". Earth and Environmental Science Transactions of The Royal Society of Edinburgh. 113 (4): 337–360. doi:10.1017/S1755691022000251. ISSN 1755-6910.
- ^ Karpinsky, Alexander (1922). "Helicoprion Ivanovi, n. sp". Bulletin de l'Académie des Sciences de Russie (Bulletin of the Russian Academy of Sciences) (in Russian): 369–379 – via Biodiversity Heritage Library.
- ^ Itano, Wayne M.; Lucas, Spencer G. (2018). "A revision of Campyloprion Eastman, 1902 (Chondrichthyes, Helicoprionidae), including new occurrences from the Upper Pennsylvanian of New Mexico and Texas, USA". Acta Geologica Polonica. 68 (3): 403–419. doi:10.1515/agp-2018-0007 (inactive 1 November 2024).
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Obruchev, D.V. (1953). "Izuchenie edestid i raboty A.P. Karpinskogo [Study of edestids and the work of A.P. Karpinsky]". Trudy Paleontologicheskogo Instituta. 45: 1–85.
- ^ a b c d e f g h i j Zangerl, Rainer (1981). Chondrichthyes I – Paleozoic Elasmobranchii. Handbook of Paleoichthyology. Stuttgart: Gustav Fischer Verlag. pp. i–iii, 1–115.
- ^ a b c d e f g h i j k Tapanila, L.; Pruitt, J.; Pradel, A.; Wilga, C.D.; Ramsay, J.B.; Schlader, R.; Didier, D.A. (2013). "Jaws for a spiral-tooth whorl: CT images reveal novel adaptation and phylogeny in fossil Helicoprion". Biology Letters. 9 (2): 20130057. doi:10.1098/rsbl.2013.0057. PMC 3639784. PMID 23445952.
- ^ a b Gayford, Joel H.; Engelman, Russell K.; Sternes, Phillip C.; Itano, Wayne M.; Bazzi, Mohamad; Collareta, Alberto; Salas‐Gismondi, Rodolfo; Shimada, Kenshu (2024). "Cautionary tales on the use of proxies to estimate body size and form of extinct animals". Ecology and Evolution. 14 (9). doi:10.1002/ece3.70218. ISSN 2045-7758. PMC 11368419. PMID 39224151.
- ^ a b c Mutter, Raoul J.; Neuman, Andrew G. (2008-01-01). "New eugeneodontid sharks from the Lower Triassic Sulphur Mountain Formation of Western Canada". Geological Society, London, Special Publications. 295 (1): 9–41. Bibcode:2008GSLSP.295....9M. doi:10.1144/SP295.3. ISSN 0305-8719. S2CID 130268582.
- ^ Engelman, Russell K. (2024-09-07). "Reconstructing Dunkleosteus terrelli (Placodermi: Arthrodira): A new look for an iconic Devonian predator". Palaeontologia Electronica. 27 (3): 1–79. doi:10.26879/1343. ISSN 1094-8074.
- ^ a b Zangerl, Rainer (17 March 1966). "A new shark of the family Edestidae, Ornithoprion hertwigi, from the Pennsylvanian Mecca and Logan quarry shales of Indiana". Fieldiana Geology. 16. Chicago Field Museum of Natural History: 1–42.
- ^ a b c d e f g h Tapanila, Leif; Pruitt, Jesse; Wilga, Cheryl D.; Pradel, Alan (2020). "Saws, Scissors, and Sharks: Late Paleozoic Experimentation with Symphyseal Dentition". The Anatomical Record. 303 (2): 363–376. doi:10.1002/ar.24046. ISSN 1932-8494. PMID 30536888.
- ^ a b c d e f Ramsay, Jason B.; Wilga, Cheryl D.; Tapanila, Leif; Pruitt, Jesse; Pradel, Alan; Schlader, Robert; Didier, Dominique A. (2 September 2014). "Eating with a saw for a jaw: Functional morphology of the jaws and tooth-whorl in Helicoprion davisii: Jaw and Tooth Function in Helicoprion". Journal of Morphology. 276 (1): 47–64. doi:10.1002/jmor.20319. PMID 25181366. S2CID 38974953.
- ^ Grogan, Eileen D.; Lund, Richard; Didier, Dominique (1999). "Description of the chimaerid jaw and its phylogenetic origins". Journal of Morphology. 239 (1): 45–59. doi:10.1002/(SICI)1097-4687(199901)239:1<45::AID-JMOR3>3.0.CO;2-S. ISSN 1097-4687. PMID 29847876. S2CID 44140851.
- ^ Lund, Richard; Grogan, Eileen D. (1997-03-01). "Relationships of the Chimaeriformes and the basal radiation of the Chondrichthyes". Reviews in Fish Biology and Fisheries. 7 (1): 65–123. doi:10.1023/A:1018471324332. ISSN 1573-5184. S2CID 40689320.
- ^ Eastman, C.R. (1900). "Karpinsky's Genus Helicoprion". The American Naturalist. 34 (403): 579–582. doi:10.1086/277706. S2CID 84684628.
- ^ Karpinsky, A. (21 November 1899). "Ueber die Reste von Edestiden und die neue Gattung Helicoprion". Bulletin de la Société Belge de Géologié, de Paléontologie et d'Hydrologie. 13 (4): 205–215.
- ^ Van den Broeck, E. (21 November 1899). "Ce que doit signifier la spirale de Helicoprion". Bulletin de la Société Belge de Géologié, de Paléontologie et d'Hydrologie. 13 (4): 215–218.
- ^ Woodward, A. Smith (19 December 1899). "Note Sur l'Helicoprion et les Edestides". Bulletin de la Société belge de géologie, de paléontologie et d'hydrologie. 13 (4): 230–234.
- ^ Simoens, G. (19 December 1899). "Note sur Helicoprion bessonowi (Karpinsky)". Bulletin de la Société Belge de Géologié, de Paléontologie et d'Hydrologie. 13 (4): 235–244.
- ^ Matsen, Bradford; Troll, Ray (1994). Planet Ocean: A Story of Life, the Sea and Dancing to the Fossil Record. Ten Speed Press. ISBN 9780898157789.
- ^ Janvier, Philippe (1998). "4.13 - Chondrichthyes". Early Vertebrates. Oxford University Press. pp. 135–150. ISBN 978-0-19-854047-2.
- ^ Purdy, Robert (February 29, 2008). "The Orthodonty of Helicoprion". NMNH Paleobiology Features. Smithsonian. Archived from the original on June 18, 2018.
- ^ Brian Switek (February 29, 2008). "Unraveling the Nature of the Whorl-Toothed Shark". Laelaps. Wired. p. 3. Retrieved September 23, 2012.



