User:Give me A+ please/sandbox
Phase I Metabolic Detoxification
[edit]Phase I metabolic detoxification, or phase I drug metabolism, is a biochemical pathway to eliminate molecules by enhancing their water solubility. The major goal is to inactivate drug molecules, but some drugs (prodrugs) are designed to be activated by phase I metabolic detoxification.[1] This process can occur in many body tissues but mostly in the liver.[2][3]
Various chemical pathways are involved in Phase I metabolic detoxification, including addition/replacement of functional groups, or bond breaking. These chemical pathways often involve enzymes to facilitate the reaction.
The rate of drug metabolism is affected by many factors, such as age and genetic profile.[2] Therefore, different individuals will have different responses to the same drug dose. External factors like drug-food and drug-drug interactions also affect the rate of metabolism.[2] The enzyme reaction site is specific to the drug substrate (lock-and-key hypothesis), so substitutions can retard the enzymatic activity, prolonging the drug's half-life.
Phase II metabolic detoxification is different from Phase I metabolic detoxification. The major phase II metabolic detoxification pathway is conjugation (attaching molecules to the drug).[1][2] Phase II metabolic detoxification can precede phase I metabolic detoxification on some occasions and not all drugs undergo both phases. They also have different enzymes involved in the pathway.
Purposes
[edit]Phase I metabolic detoxification is a physiological pathway that eliminates drugs in the body via chemical pathways, usually with the help of enzymes. Possible results of this process include inactivating active drugs and activating prodrugs.[1]
Drug inactivation
[edit]Some drugs’ bioactivity is terminated by phase I metabolic detoxification. The metabolite will become more hydrophilic (water-loving or water-soluble).[1][2] Toxic metabolites may also be formed during this process, and they can damage body tissues.[2] An example is the toxic metabolite of paracetamol, NAPQI, which can damage liver cells. Therefore, liver failure can occur due to severe paracetamol overdose.[4] As a result, the maximum suggested dose of paracetamol for adults is four grams per day.[4]
Prodrug activation
[edit]Prodrugs are inactive and only become active after metabolism. They are designed to increase the lipophilicity of the drug so it can penetrate the cell membrane (which is lipophilic) of the small intestine and be absorbed into the bloodstream, improving oral absorption.
Active metabolite
[edit]Some active drugs can be metabolized without termination of bioactivity, forming an active metabolite and prolonging the duration of action.
Outcome
[edit]The ultimate outcomes of metabolic detoxification are to increase water solubility (hydrophilicity) and reduce the bioavailability of drug molecules. The increase in water solubility favours their dissolution in urine and removal from the body through urine excretion.
Reaction Sites
[edit]The major enzyme in phase I metabolic detoxification, cytochrome P450 (CYP450), is located in the smooth endoplasmic reticulum, an organelle inside human cells.[2] Drug metabolism can occur in every tissue, some examples being the kidney and blood plasma. However, the liver is the major site because it has more enzymes than other tissues.[2][3] Acute or chronic diseases affect liver function, for example, liver failure can reduce enzyme production and alter enzyme activity. Depending on their severity, these conditions may significantly impair hepatic drug-metabolizing enzymes like CYP450s. Consequently, drugs may accumulate in body, causing overdosing toxicity.[1] As a result, the dose of drugs metabolised by the liver have to be adjusted.[5]
Metabolic Pathway
[edit]The common chemical pathways for phase I metabolic detoxification include reduction (the gain of electrons), oxidation (the most common type for phase I metabolic detoxification; the loss of electrons), and hydrolysis (the breakdown of bonding with water).[1][2]
Metabolic reactions can alter the shape of molecules to convert them into inactive drug forms, thus reducing the amount of active drug molecules for pharmacological actions. Furthermore, the structure of molecules can also be converted to a more active one. Prodrugs are designed to be active after this pathway, so undergoing metabolic pathway helps increasing the amount of active drugs instead.
A drug molecule can be metabolized in different pathways. Even the same metabolic pathway may result in different products. Therefore, the mechanism of phase I metabolic detoxification is used to predict possible metabolites. Most phase I metabolic detoxification reactions require the CYP450 enzyme system.[1][2][3] The presence of enzymes aid the reaction rate by lowering the energy required for the initiation of reactions in the pathways. After metabolism, the molecule will become more hydrophilic.
Side chain (R) means any atoms or group of atoms apart from hydrogen (H) on this page.
Primary, secondary and tertiary carbons refer to carbon atoms attached to one, two and three side chain(s) respectively. Similarly, primary, secondary and tertiary amines refer to nitrogen atoms attached to one, two and three side chain(s) respectively.
Oxidation
[edit]Metabolism of C–H bonds: C−H Abstraction, followed by Hydroxylation
[edit]Most drugs contain C–H bonds, which will be metabolized to C–OH bonds. Enzyme CYP450s will attack the C–H bond, forming a carbon radical with the loss of a hydrogen atom, which is described as an ‘intermediate step’ or ‘C−H Abstraction’. A hydroxyl (-OH) group will be added to this radical, catalyzed by the same enzyme, CYP450s. As a result, this process is called ‘hydroxylation’.[1][3]
Metabolism of non-aromatic C=C bonds: dihydroxylation
[edit]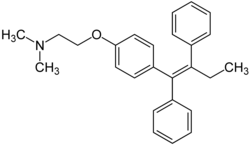
At least one hydrogen atom must be present on one of the two alkene carbon atoms. For example, the C=C bond of tamoxifen cannot undergo oxidation as there is no hydrogen atom on any carbon atom of the C=C bond.[1]
C=C bond reacts to form epoxide with the aid of the CYP450s, followed by an addition of a water (H2O) molecule. The overall result is two hydroxyl (-OH) groups being inserted into the C=C bond, with the C=C bond becoming a C–C bond.[1] As a result, this process is named ‘dihydroxylation’.
Metabolism of aromatic rings: aromatic hydroxylation
[edit]

In the aromatic ring, a C−H bond is oxidized to a C−OH bond, which differs from the dihydroxylation of C=C bonds.[1][3] This is described as ‘aromatic hydroxylation’.
The preferred position for aromatic hydroxylation in the aromatic ring is the para position over the meta position. The ortho position is the least preferred site for aromatic hydroxylation.
Metabolism of amines: N-oxidation and dealkylation
[edit]
Primary amine (RNH2) will be metabolized to nitroso (RN=O). Secondary amine (R2NH) will be metabolized to hydroxylamine (R2NOH). Tertiary amine (R3N) will be metabolized to N-oxide (R3N+→O-). An oxygen atom is added to the tertiary amine, where a dative covalent bond is formed between the nitrogen and oxygen atoms. The enzyme involved in this reaction can be either CYP450s or flavin-containing monooxygenase (FMO).[1][3]

Amine can also undergo dealkylation sequentially. The tertiary amine is metabolized by step to secondary and primary amines respectively. The primary amine can be further dealkylated to become ammonia (NH3). This process is also called ‘deamination’.[1][3] The carbon atom connected to the nitrogen atom must be bonded with at least one hydrogen atom for dealkylation to occur.[1]
Metabolism of other functional groups
[edit]| Reaction name | Reactant's functional group | Enzymes required | Product's functional group | Remarks |
| Oxidation of 2° | Secondary alcohol (two side chains; R2CHOH) | Alcohol dehydrogenase (ADH)[1] | Ketone group (R2C=O) | / |
| Oxidation of 1° | Primary alcohol (one side chain; RCH2OH) | ADH then aldehyde dehydrogenase (ALDH)[1] | Aldehyde group (RCHO) then to carboxylic acid (RCOOH) | ADH aids the transformation of primary alcohol to aldehyde, and ALDH catalyzes the reaction of aldehyde to carboxylic acid.[1] |
| Dealkylation | Ether group (ROCR) | CYP450s[1] | Hydroxyl group (ROH) | The ether group undergoes dealkylation and the other product is the aldehyde group.[1][3] |
| Dealkylation | Thioether (RSCR) | CYP450s[1] | Thiol group (RSH) | Similar to ether. The other product is the aldehyde group.[1][3] |
| Dimerization | Thiol group | FMO[1] | Disulphide (RS-SR) | Two thiol groups join together.[1][3] |
| S-oxidation | Sulphide group (R2S) | FMO[1] | Sulphoxide (R2SO) then sulphone (R2SO2) subsequently | Similar to N-oxidation. |
| Desulfuration | Thiocarbonyl group (R2CS) | CYP450s[1] | Ketone group (R2CO) | An aldehyde group will be formed if one of the side chains is a hydrogen atom.[1] |
Reduction
[edit]Some of the reduction reactions are the reverse of oxidation reactions. All of the following enzymes require NADPH as the reducing species.[1]
| Reaction name | Functional group | Enzymes required | Metabolized group | Remarks |
| C=O reduction | Ketone group | Aldo-keto reductase[1] | Secondary alcohol | Reverse of the oxidation of 2° alcohol. |
| C=O reduction | Aldehyde group | Aldo-keto reductase[1] | Primary alcohol | Reverse of the oxidation of 1° alcohol. A minor route comparing to ALDH oxidation.[1] |
| S−S reduction | Disulphide | / | Thiol group | Reverse of dimerization. |
| S=O reduction | Sulphoxide | / | Sulphide group | Reverse of S-oxidation. |
| N=N reduction | Azo group (RN=NR) | Azoreductase[1] | Amine group | N=N bond will be cleaved. |
Graphical summary of oxidation and reduction of other functional groups
[edit]
Hydrolysis
[edit]Hydrolysis can also occur in the ester (RCOOR) and amide (RCONH2) groups. With the addition of a water (H2O) molecule, ester will be cleaved into carboxylic acid and alcohol, while amide will be cleaved into carboxylic acid and amine. The enzymes responsible for these reactions are esterase and amidase respectively.[3][1]

Strategy to prevent metabolism: substitution
[edit]
Enzymatic actions occur through the binding of drug molecules (substrate) to the enzymatic site. By the lock-and-key hypothesis, the substrate is specific to the enzymatic pocket. Therefore, the substitution of a drug can affect the enzyme’s ability to recognize it. Hence, the drug’s half-life (the time required for a drug’s concentration to drop to 50% of its original amount) can be prolonged.

For example, chlorpheniramine and triprolidine have similar structures, but chlorpheniramine has a chlorine substituent instead of a methyl substituent at the benzene ring. It is seen that chlorpheniramine has a half-life of 21 to 27 hours [7] while that of triprolidine is 4 to 6 hours,[8] which indicates chlorpheniramine has a more sustained effect and is less readily metabolized. This results in the dosing frequency of chlorpheniramine (once per day) [9] being lower than triprolidine (four times per day).[10] Therefore, a common strategy to increase the half-life of a drug is to insert a chlorine atom into the benzene ring.
Factors Affecting Drug Metabolism
[edit]Alteration of enzyme activity
[edit]As phase I metabolism of drugs requires different enzymatic activities, its rate can be directly influenced by inhibiting or inducing the enzymes.
Enzyme inhibitors
[edit]
When the enzymes responsible for drug metabolism are inhibited, for example, through binding with other substances, the drugs will be less metabolised. Inhibition can be categorised as reversible and irreversible, both of which lead to the accumulation of drug molecules. For instance, grapefruit juice can inhibit CYP3A4, the major isoenzyme of CYP450s superfamily, which is responsible for the phase I metabolism of many drugs. It is found that furanocoumarins, ingredients of grapefruit juice, inhibit the metabolism of simvastatin.[11] The metabolism of simvastatin consists of C−H abstraction and, subsequently, hydroxylation to the carbon radical to form the C−OH hydroxy metabolite, which is catalyzed by CYP3A4. The metabolites are subsequently metabolized into inactive forms. By inhibiting CYP3A4, grapefruit juice greatly reduces the extent of phase I metabolism of simvastatin into its intermediate metabolites, thus, further metabolism cannot proceed normally. Therefore, it is usually advised for patients taking simvastatin to refrain from drinking grapefruit juice. Inhibition of esterase is another example. Esterase is responsible for phase I metabolism of an antihypertensive prodrug, enalapril, into its active form, enalaprilat. Therefore, if esterase is inhibited, less-active enalapril will accumulate without significantly changing to its active metabolites. This reduces its function of lowering blood pressure.
Enzyme inducers
[edit]Enzymatic induction refers to the increased activity of the metabolic enzymes. Therefore, more drugs will be converted to their metabolized form. It takes a longer time to occur than enzymatic inhibition.
For example, polycyclic aromatic hydrocarbons (i.e. benzo[a]pyrene) in tobacco smoke induce the liver enzyme CYP1A2, which metabolizes clozapine into its inactive metabolite. Thus, blood concentrations of clozapine decrease,[12] as seen from the increased plasma concentration of clozapine in smokers. Clozapine has a narrow therapeutic window (i.e., having a narrow dose range in which the drug is both safe and effective). When the clozapine level in the blood is reduced by smoking-induced metabolism, the possibility of it producing subtherapeutic effects is increased. Therefore, lowering the dose of clozapine warrants more attention, as the therapeutic window of this drug is narrow.
Other factors affecting drug metabolism
[edit]The following lists some factors affecting drug metabolism.
| Factors | Mechanism | Result |
| Genetic Profile | Metabolic enzyme isoforms alter enzyme activity to process drugs or prodrugs. People with higher enzymatic activities are called fast metabolizers; those with lower activities are called slow metabolizers[3] | Fast metabolizers require higher doses of the same medication; slow metabolizers require lower doses of the same medication. The case is reversed for prodrugs. |
| Age and Nutrition | The amount of metabolic enzymes generally decreases with age. Children may also be deficient in enzymes as their enzymatic organs are in the growth stage. | Children and the elderly may require special dosing (e.g., for children, acetaminophen is given in the dose based on weight and age) [5] |
| Dosage | Enzymes are occupied by the drugs when they are used for metabolism, thus inhibiting them from metabolizing other intact drugs before the ongoing metabolism is finished. | An extremely high amount of drugs saturates the enzymes and renders metabolism of the excess amount of drugs. |
| Route of Administration | If drugs are taken orally, they will reach the liver through the oral route and thus undergo hepatic first-pass metabolism, which decreases the amount of drug reaching their site of action, as the liver is a major metabolic enzyme. Drugs delivered through intravenous injections bypass the liver and thus liver first-pass metabolism.[13] | Some drugs may only be injected intravenously due to them being extensively metabolised by the liver. |
| Other Drugs/Food | Some drugs or substances in food use the same enzymes for metabolism, rendering the enzymes from metabolising multiple drugs or substances at the same time. | Some foods or other drugs are contraindicated with the drugs prescribed. Otherwise, toxicity of the prescribed drugs due to overaccumulation of drugs in the body may occur. |
Comparison between Phase I and Phase II Metabolic Detoxification
[edit]The following table briefly compares phase 1 and phase 2 metabolic detoxification.[2][1]
| Phase I metabolic detoxification | Phase II metabolic detoxification | |
|---|---|---|
| Action on drugs | Functional group transformation: changing the functional group from the original one to another | Conjugation: attaching another group to the original compound |
| Order | Phase II may precede phase I | |
| Metabolic enzymes involved |
Oxidation[edit]
Reduction[edit]
Hydrolysis[edit]
|
|
| Purpose |
| |
Wordcount: 1681 words
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai Harrold, M. W., & Zavod, R. M. (2023). Basic concepts in medicinal chemistry (3rd ed.). ASHP.
- ^ a b c d e f g h i j k Foye, W. O., Lemke, T. L., & Williams, D. A. (2013). Foye’s principles of medicinal chemistry (7th ed.). Wolters Kluwer Health/Lippincott Williams & Wilkins.
- ^ a b c d e f g h i j k l m Katzung, B. G., & Vanderah, T. W. (Eds.). (2021). Basic & clinical pharmacology (15th ed.). McGraw-Hill.
- ^ a b Acetaminophen (paracetamol): Drug information. UpToDate. (n.d.). https://www.uptodate.com/contents/acetaminophen-paracetamol-drug-information?search=paracetamol+&source=panel_search_result&selectedTitle=1~150&usage_type=panel&kp_tab=drug_general&display_rank=1
- ^ a b Joint Formulary Committee (2024). British National Formulary (87th ed.). London, UK: BMJ and Pharmaceutical Press (published 20 March 2024). ISBN 978-0857114808.
- ^ "Blue Book chapter P-1". iupac.qmul.ac.uk. Retrieved 2025-03-09.
- ^ "Chlorpheniramine". go.drugbank.com. Retrieved 2025-03-09.
- ^ "Triprolidine". go.drugbank.com. Retrieved 2025-03-09.
- ^ "Chlorphenamine: drowsy (sedating) antihistamine". nhs.uk. 2018-09-20. Retrieved 2025-03-09.
- ^ "Triprolidine: Uses, Dosage, Side Effects and More | MIMS Hong Kong". www.mims.com. Retrieved 2025-03-09.
- ^ Guo, Yamazoe, Lian-qing, Yasushi. "Inhibition of Cytochrome P450 by furanocoumarins in grapefruit juice and herbal medicines" (PDF). Acta Pharmacologica Sinica.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Taylor, David (Aug 1998). "Pharmacokinetic interactions involving clozapine". The British Journal of Psychiatry: The Journal of Mental Science. 171. doi:10.1192/bjp.171.2.109. ISSN 0007-1250. PMID 9337943.
- ^ Ronco, Bellomo, Kellum, Ricci, Claudio, Rinaldo, John, Zaccaria (2017). Critical Care Nephrology (3rd ed.). Elsevier (published 5 Dec 2017). ISBN 9780323449427.
{{cite book}}: CS1 maint: multiple names: authors list (link)

