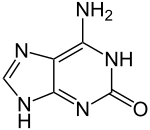
Isoguanine
 | |
| Names | |
|---|---|
| Preferred IUPAC name
6-Amino-1,9-dihydro-2H-purin-2-one | |
| Other names
2-Hydroxyadenine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.020.144 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H5N5O | |
| Molar mass | 151.1261 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isoguanine or 2-hydroxyadenine is a purine base that is an isomer of guanine. The nucleoside form is called isoguanosine (iG).[1][2]
It is a product of oxidative damage to DNA in cultured E. coli and human cells. However, newer evidence shows that it mainly occurs in RNA and as the ribonucleoside instead of in DNA or as deoxyribonucleoside in living mammals, calling into question its link with DNA in vivo.[2]
It can be mutagenic by causing mispairing, but does not hinder replication.[3][2]

It is also used in combination with isocytosine in studies of unnatural nucleic acid analogues of the normal base pairs in DNA.[4][5] It is used as a nucleobase of hachimoji nucleic acids where it is given the abbreviation B.[6] In hachimoji DNA, it pairs with 1-methylcytosine, while in hachimoji RNA, it pairs with isocytosine.
References
[edit]- ^ Hopfinger, MC; Kirkpatrick, CC; Znosko, BM (18 September 2020). "Predictions and analyses of RNA nearest neighbor parameters for modified nucleotides". Nucleic acids research. 48 (16): 8901–8913. doi:10.1093/nar/gkaa654. PMC 7498315. PMID 32810273.
- ^ a b c Ding, Tingting; Tang, Fan; Ni, Guangcheng; Liu, Jiang; Zhao, Hang; Chen, Qianming (2020). "The development of isoguanosine: from discovery, synthesis, and modification to supramolecular structures and potential applications". RSC Advances. 10 (11): 6223–6248. doi:10.1039/C9RA09427J.
- ^ Yang XL, Sugiyama H, Ikeda S, Saito I, Wang AH (1998). "Structural studies of a stable parallel-stranded DNA duplex incorporating isoguanine:cytosine and isocytosine:guanine basepairs by nuclear magnetic resonance spectroscopy". Biophys. J. 75 (3): 1163–1171. Bibcode:1998BpJ....75.1163Y. doi:10.1016/S0006-3495(98)74035-4. PMC 1299791. PMID 9726918.
- ^ Andrzej Jaworski, Józef S. Kwiatkowski, Bogdan Lesyng: „Why isoguanine and isocytosine are not the components of the genetic code", International Journal of Quantum Chemistry, Supplement: Proceedings of the International Symposium on Quantum Biology and Quantum Pharmacology, 1985, 28 (Supplement S12), pp. 209–216 (doi:10.1002/qua.560280720).
- ^ Christopher Roberts, Rajanikanth Bandaru, Christopher Switzer: „Theoretical and Experimental Study of Isoguanine and Isocytosine: Base Pairing in an Expanded Genetic System", J. Am. Chem. Soc., 1997, 119 (20), pp. 4640–4649 (doi:10.1021/ja970123s).
- ^ Hoshika, Shuichi; et al. (22 February 2019). "Hachimoji DNA and RNA: A genetic system with eight building blocks". Science. 363 (6429): 884–887. Bibcode:2019Sci...363..884H. doi:10.1126/science.aat0971. PMC 6413494. PMID 30792304.
