Bopindolol
 | |
| Clinical data | |
|---|---|
| Trade names | Sandonorm |
| Other names | LT 31-200 |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| Drug class | Beta blocker |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H28N2O3 |
| Molar mass | 380.488 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Bopindolol (INN), sold under the brand name Sandonorm among others, is a beta blocker used to treat hypertension.[1][2][3][4] It has been marketed in a number of countries throughout the world, for instance in Europe.[2]
Pharmacology
[edit]Bopindolol is an ester prodrug of mepindolol.[3][4] It acts as a non-selective or dual β1- and β2-adrenergic receptor antagonist.[4] Bopindolol has intrinsic sympathomimetic activity (ISA) and membrane-stabilizing activity (MSA).[4] Besides the β1- and β2-adrenergic receptors, bopindolol shows very low affinity for the β3-adrenergic receptor and interacts with certain serotonin receptors such as the serotonin 5-HT1A receptor with strong affinity.[4]
Chemistry
[edit]The predicted log P of bopindolol ranges from 4.45 to 4.7.[5][6] It showed the highest predicted lipophilicity of 30 clinically relevant beta blockers, with the second most lipophilic beta blocker predicted to be the better-known penbutolol.[7]
Synthesis
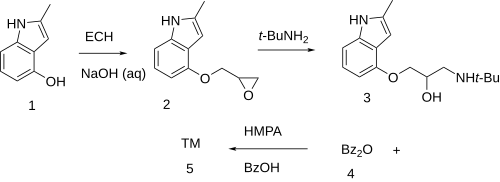
[edit]The reaction of 4-Hydroxy-2-methylindole [35320-67-3] (1) with epichlorohydrin in the presence of lye led to 2-methyl-4-(oxiran-2-ylmethoxy)-1H-indole [62119-47-5] (2). Addition of tert-butylamine led to 4-(2-Hydroxy-3-tert-butylaminopropoxy)-2-methylindole [23869-98-9] (3). Ester formation with benzoic anhydride [93-97-0] (4) in the presence of hexamethylphosphoric acid triamide [680-31-9] completed the synthesis of Bopindolol (5).
History
[edit]Bopindolol is related to and was developed as a successor to pindolol.[4] It was first described in the literature by at least 1977.[1]
References
[edit]- ^ a b Elks, J. (2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer US. p. 169. ISBN 978-1-4757-2085-3. Retrieved 10 July 2025.
- ^ a b Schweizerischer Apotheker-Verein (2004). Index Nominum: International Drug Directory. Index Nominum: International Drug Directory. Medpharm Scientific Publishers. p. 154. ISBN 978-3-88763-101-7. Retrieved 10 July 2025.
- ^ a b Morton, I.K.; Hall, J.M. (2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Netherlands. p. 53. ISBN 978-94-011-4439-1. Retrieved 10 July 2025.
- ^ a b c d e f Nagatomo T, Hosohata Y, Ohnuki T, Nakamura T, Hattori K, Suzuki J, Ishiguro M (Spring 2001). "Bopindolol: pharmacological basis and clinical implications". Cardiovascular Drug Reviews. 19 (1): 9–24. doi:10.1111/j.1527-3466.2001.tb00180.x. PMID 11314603.
- ^ "Bopindolol". PubChem. Retrieved 10 July 2025.
- ^ "Bopindolol: Uses, Interactions, Mechanism of Action". DrugBank Online. 20 October 2010. Retrieved 10 July 2025.
- ^ Mannhold R (February 2005). "The impact of lipophilicity in drug research: a case report on beta-blockers". Mini Rev Med Chem. 5 (2): 197–205. doi:10.2174/1389557053402701. PMID 15720289.
- ^ DE2635209 idem Franz Troxler, Fritz Seemann, U.S. patent 4,434,176 (1984 to Sandoz Ltd.).
- ^ Franz Dr Troxler & Albert Dr Hofmann, CH453363 (1968 to Sandoz AG).
